As the primary source of vision care and guardians of eye health for millions, optometrists play a critical role in diagnosing and managing diabetic retinopathy. They decide who needs regular follow-ups, whom should be referred out to specialists, and for those patients with a lower risk—whom should be monitored at more frequent intervals. This role comes with a great deal of responsibility, and if eyecare professionals don’t have
the right tools, it's much more challenging to safeguard against disease progression and vision loss.
What is the impact of diabetic retinopathy?
A major concern for today’s eyecare professionals, diabetic retinopathy (DR) is now the leading cause of blindness in working-age Americans and is a major cause of blindness worldwide. According to the Centers for Disease Control and Prevention (CDC), there are 130 million adults living with diabetes or prediabetes in the US, as outlined in the
2022 National Diabetes Statistics Report.
In addition to potentially damaging the heart and liver, high blood sugar levels from diabetes can damage blood vessels in the retina. The vessels might swell and leak (non-proliferative diabetic retinopathy), or they could close, stopping blood from passing through and reaching the macula (ischemia).2 Alternatively, abnormal, new blood vessels might grow on the retina (proliferative diabetic retinopathy). These fragile, new vessels often bleed into the vitreous, causing floaters or vision loss. Ultimately, these abnormal, new vessels can form scar tissue, leading to retinal detachment and irreparable vision loss.2
According to 2020 estimates by the American Society of Retina Specialists, diabetic retinopathy affects about 8 million Americans. That number is expected to double by 2050,3 and could be costing the nation $500 million annually. This might seem outrageous until you consider that about one-third of adults over age 40 have diabetes, and more than one-third of Black and Mexican-Americans are diagnosed with this condition. (Diabetic retinopathy is more than twice as common in Mexican-Americans and almost three times as common in Blacks.4,5) Diabetes also leads to earlier development of cataracts and some studies suggest it increases the risk of glaucoma.6
“The mission at my practice is prevention of vision loss,” said Dorothy Hitchmoth, OD, FAAO, who practices in New London, New Hampshire. “One of the only ways you can do that is to catch disease in its earliest stages, especially retinal disease. Now, we know that you can change the trajectory of macular degeneration, diabetic retinopathy, and glaucoma. So having the tools that help doctors detect those diseases early is critically important.”
“I see patients every day with diabetes and diabetic retinopathy,” said A. Paul Chous, MA, OD, FAAO. Dr. Chous is an optometrist and diabetes educator at Chous Eyecare in University Place, Washington and the majority of his patients have diabetes. “Patients can be totally asymptomatic,” said Dr. Chous. “They can have great vision until the day they don’t.” He adds: “A fair number of people—about 10%—who have been identified as having pre-diabetes already have diabetic retinopathy. So you can’t assume because someone has pre-diabetes that they don’t already have eye damage. Most diabetes specialists consider ‘pre-diabetes’ as an early stage of type 2 diabetes.”
Like many eye diseases, diabetic retinopathy may not cause symptoms, especially in the early stages. Some patients may notice changes in their vision or have trouble seeing objects in the distance, but by the time patients see dark floating spots or streaks, it’s usually because the disease has advanced to where the blood vessels in the retina are damaged or start to bleed into the vitreous.7
Diabetic retinopathy can also lead to neovascular glaucoma, where abnormal blood vessels grow on the eye’s internal drainage canal and block aqueous from draining out of the trabecular meshwork, which causes a dramatic increase in intraocular pressure. At advanced stages, neovascular glaucoma can cause total blindness.
Benefits and limitations of structural imaging
Structural imaging is key to understanding the anatomical changes underlying diabetic retinopathy. Historically, fundus photography and optical coherence tomography (OCT) provided insight about DR through images of the retinal vasculature, optic nerve, and a limited view of the choroid. But while these are both important as part of a work-up, they may not be enough because subtle vascular lesions may be undetectable or missed due to image resolution.
OCT is likewise useful and increasingly relied upon for measuring macular and peripapillary thickness, which can provide quantitative data that can be used to diagnose and monitor disease progression. The downside is that clinical maps may provide a one-size-fits-all representation8 and can be limited by opacities from light waves interfering with optimal imaging. Additionally, OCT images can be impacted in the setting of vitreous hemorrhage, dense cataract, corneal opacities, and patient movement.
The benefits of functional analysis in optometry
In short, while fundus photography and OCT each provide important two- and three-dimensional insights into structural changes of the retina, functional changes often precede structural changes in many eye diseases, including diabetic eye disease.9 For example, the prediabetic stage reveals earliest functional neuronal changes in the retina. In diabetes patients in general, the neuronal function seems to be affected much earlier than clinically appreciable structural changes in the ganglion cell complex and precedes vascular changes in the retina. Detecting these functional abnormalities before structural damage is observed can facilitate the timely management of ophthalmic disorders.10
Additionally, functional analysis delivers information on alterations in biological activities including metabolism, blood flow, regional chemical composition, and biochemical processes.11 Structural imaging cannot achieve this level of functional insight, in part, because it is so difficult to obtain images from the ‘dark corners’ of the posterior segment.
For example, regional retinal nerve fiber layer (RNFL) damage quantifiable at the optic nerve head maps to large areas of the retina, including wide tracts outside the region tested by standard assessment of the central visual field. Furthermore, in the macula, the retinal ganglion cells are displaced from their photoreceptors, leading to a spatial shift between retinal ganglion cell damage reported on OCT and corresponding visual field locations.12
Visual acuity and perimetry limitations
In an ideal world, the complete assessment of vision-related abilities should consider visual function (the performance of components of the visual system) and functional vision (visual task-related ability). The problem with current assessment methods is that they are often highly dependent upon individual characteristics (e.g. the presence and type of visual impairment).
Visual function describes how well the eyes and basic visual system can detect a target stimulus. By varying a single parameter at a time (for example, the size of the target), testing is typically carried out in a repeated fashion under controlled testing conditions until a threshold of performance is obtained. In contrast, functional vision refers to how well an individual performs while interacting with the visual environment. Typical visual function tests assess factors such as visual acuity, contrast sensitivity, color, depth, and motion perception. These properties each represent an aspect of visual function and may impact an individual’s level of functional vision.13
However, these tests are subjective, and reliable results depend on patients’ optimal performance, including vigilance, during a test.14 Other disadvantages of some visual field tests include that the large targets sometimes miss small, early defects, particularly in the paracentral region.15 This is why an objective measure of function is so valuable.
Despite the availability of modern imaging tools, many patients with diabetic retinopathy remain undiagnosed. Visual field and visual acuity tests rely on patient feedback. What’s needed is a more objective measure of function, like electrophysiology (e.g. electroretinography), which provides objective information on the function of the visual system.
Electroretinography basics
While structural imaging shows vascular abnormalities,
electroretinography (ERG) objectively evaluates functional abnormalities by measuring the electrical activity of the retina. Specifically, an electroretinogram (ERG) is a diagnostic test that utilizes light stimulus to generate a measurable electrical response in various cell types in the retina, including the photoreceptors (rods and cones), inner retinal cells (bipolar and amacrine cells), and the ganglion cells. The ERG arises from currents generated directly by retinal neurons in combination with contributions from retinal glia.
The ERG is to the retina what the electrocardiogram (ECG) is to the heart. Just as an ECG is crucial to diagnosing illness and monitoring the heart’s function, ERG plays a critical role in the care of the eye, and is instrumental in the early detection of retinal dysfunction. Electroretinography provides a wealth of objective information about retinal function and health and plays an invaluable role in the diagnosis of acquired and inherited eye diseases.
Why electroretinography benefits diabetic retinopathy patients
Functional changes have been shown to precede structural changes seen via dilated eye examination or retinal imaging, especially in prediabetes.
16 For patients who might have diabetic retinopathy—in which early detection is crucial to vision preservation—functional testing may facilitate earlier detection and treatment that could alter a patient’s quality of life for decades. Furthermore, research has suggested that ERG testing may have the highest predictive value in
forecasting the progression of diabetic retinopathy.17, 18As the leading cause of blindness in working-age adults, diabetic retinopathy is typically asymptomatic until patients experience significant structural damage, long after the disease initially develops. For this reason, an objective test that provides optometrists with clear metrics can help with decision-making and provide insight into whether a patient can be managed in-house or should be referred out to an ophthalmologist or retinal specialist.
How to interpret an ERG result
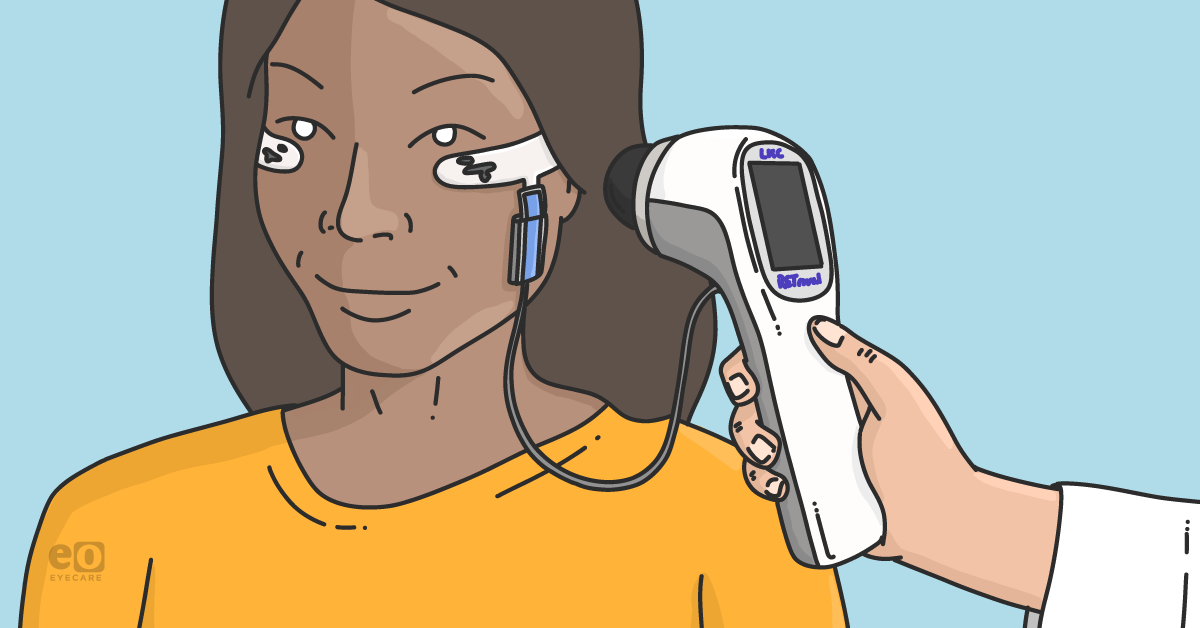
Modern, handheld ERG technology is nothing like the wires and corneal electrodes that were used in labs in decades past. Today’s technology is supremely simple and practical, offering very clear reporting. For example, the
RETeval® device is handheld, portable, and can test retinal function non-invasively, quickly, and prior to clinical examination through undilated pupils. It is appropriate for all patients, at any age, without any need for sedation or special training. In fact, technicians can easily obtain results for interpretation by eye doctors in conjunction with clinical examination.
“The RETeval device is my new favorite tool, and that’s no exaggeration,” said Dr. Hitchmoth. “It’s highly accurate and it literally takes minutes. I can do it, my technicians can do it, and I would say it’s easy enough for a medical assistant to use. There’s not a lot of other tests I can say that about.”
Utilizing patented, non-invasive sensor strips, the RETeval tool delivers precise data on retinal function by measuring both retinal cell stress and pupil light response. As a result, the RETeval device provides information needed to make better decisions and to understand which patients should be seen more often.
Mike Cymbor, OD, FAAO, practices optometry at Nittany Eye Associates in Pennsylvania and uses the RETeval device in his practice. “It can be difficult to get objective test results from visual field testing if a patient isn’t feeling well,” he said. “The RETeval device takes that out of the equation, and can help an eyecare professional objectively evaluate how a patient should be managed and treated.”
The RETeval device is most commonly used to aid in the diganosis of:
How does the RETeval device work?
The
RETeval device will flash a series of lights into the patient’s eyes. The retina then responds to the flashes by generating small, electrical signals that travel through the facial structure to the sensor strips placed on the lower eyelid. When following the diabetic retinopathy protocol, RET
eval sensor strips detect the electrical signals and will compare the results to an age-adjusted diabetic retinopathy reference database. Specifically, a diabetic retinopathy assessment takes the following factors into account:
- Implicit time for the retina to respond (ERG)
- Amplitude of response (ERG)
- Pupil response and change in diameter from dim to bright
- Patient age
“I like that the RET
eval uses a
numerical score combined with red, yellow, and green color coding” said Dr. Hitchmoth. “And the score gives me two major things. One, I can differentiate diseases, and two, I can detect abnormalities sooner.”
RETeval assesses DR risk with a global score that evaluates the amplitude and timing of the retinal response to the stimuli, as well as pupillary response to those same stimuli. A DR Score of:
- 23.5 and above denotes an 11-fold increase in the likelihood that the patient will require a retinal intervention (laser or anti-VEGF) within 3 years19 while 23.4 and below does not.
- A score of 26.0 and higher is predictive of a patient needing treatment within a year.
“I like tests that are objective, particularly from a patient standpoint because it’s hard to create a bad answer from objective information,” said Jeffry Gerson, OD, who practices at Grin Eye Care in Kansas. “The RETeval device gives you objective information that helps you solve clinical puzzles.”
When should optometrists use the RETeval device?
“If I see a patient with newly diagnosed diabetes who also has poor metabolic control, and I suspect the diabetes has been going on for a long time, then I want to use the RETeval device to get a baseline reading of current retinal function and future risk,” said Dr. Chous.
“Once I know a patient has had diabetes for a number of years,” added Dr. Cymbor, “and I suspect diabetic macular ischemia even without seeing conventional changes on structural imaging, I add ERG testing with the RETeval device. Looking at structural imaging of patients with diabetic retinopathy—or whom you expect may have it—can be confusing even for experienced practitioners. Results from the RETeval help me understand the sweet spot of when I need to bring a patient back in and when I should seek intervention from a retinal specialist.”
How can RETeval help you care for diabetic retinopathy patients?
“The biggest benefit of the RETeval device for me is understanding whether or not a patient has damage in their retina that I cannot see, and to share that with the patient,” said Dr. Hitchmoth.“ Then I can initiate a treatment plan and tell a patient exactly what they need to do. Additionally, once I understand their risk, I know when I should see them next and how to best monitor them to achieve the most successful outcome.”
“It used to be that retinal function was evaluated by visual acuity," said Dr. Cymbor. "But now we know that functional damage can occur long before visual acuity is affected. The RETeval device makes that possible for us in a more efficient way than ever before. We now know how important retinal health is to measuring visual function and ERG using the RETeval device can do that.”
A 2020 study examined 279 diabetic patients who were not previously treated for either macular edema or diabetic retinopathy.20 This longitudinal study demonstrated the ability of the RETeval device to predict progression of retinopathy. It likewise showed that combining structure and function provides a more comprehensive analysis to make clinical decisions and understand when to manage a patient internally or refer out. In studies comparing ERG and structural imaging’s abilities to evaluate sight-threatening diabetic retinopathy, RETeval ERGs outperformed the traditional imaging techniques in predicting which patients would later need medical intervention.21,22
“Let’s say a patient has more than a few microaneurysms, they’ve got some intra-retinal hemorrhage and maybe some hard exudates without OCT-confirmed DME,” continued Dr. Chous. “If I do a RET
eval analysis and their
DR Score is below the threshold of 23.5, I feel good about seeing them in six months, because their functional testing is showing me their retina is not that sick—but I want to follow up to monitor change over time. Now let’s turn that on its head and say the score is a 25 or 26. That patient is at significantly higher risk—like 11-fold higher risk—for developing retinopathy or DME of a severity that requires treatment. So I’m going to talk to that patient about improving their metabolic control and see them much sooner to monitor them than I would have from a structural test alone, and depending on disease severity, I will likely refer them to a retinal specialist.”
As mentioned above, the
RETeval test gives optometrists a clear score to determine a patient’s DR risks. A score of 23.5 or higher indicates an 11-fold risk of requiring intervention within three years.
23 Dr. Chous explains why knowing this is so important:
“We know that a mere 2.8 unit change in the diabetic retinopathy score doubles the risk of a patient developing retinopathy to the stage where they will need treatment within three years. There’s a threshold at which the risk of needing intervention goes way up. What excites me about the RETeval device is that it gives optometrists who aren’t sure about the degree of severity an opportunity to see if their opinion is in line with retinal function. They can see if they might need to follow up with a patient more closely than what was detected from clinical exam or structural imaging.”
Dr. Chous is excited about the potential of functional testing using the RETeval device because it provides insights into disease progression and empowers doctors by providing the information needed to make confident, informed choices.
Case in point: “I just saw new data presented at an electrophysiology conference in Asia that showed if a RETeval score was above 26.9, the likelihood of developing proliferative diabetic retinopathy or diabetic macular edema was more than seven times higher than if you just looked at fundus photos,21” said Dr. Chous. “Fundus photography was totally uncorrelated with the likelihood of disease progression, which really shows the power of the RETeval device. The next closest predictor was ultra-widefield fluorescein angiography with ischemia measurements—but even that was only half as effective as RETeval for identifying patients at higher risk of rapid progression to sight-threatening disease.”
How can the RETeval device impact ROI?
Generally, a return on investment includes the amount of reimbursement minus the cost of the test and the level of disruption. As mentioned earlier, this testing takes only minutes to complete and is simple to perform and interpret. In addition, Medicare does not consider this test mutually exclusive to other diagnostic tests.
“This means that you can perform ERG, visual fields and other photo-imaging on the same day,” says Dr. Hitchmoth. “Fewer visits at a higher revenue per encounter results in improved margins and less inconvenience to patients who are in need of testing.”
To further simplify integrating ERG into practice, Dr. Hitchmoth recently partnered with LKC to develop a
billing and coding guide. “Remarkably, there are 560 ICD-10 codes to choose from, but the simple
coding guide will help make chair-side investigations simple,” she says.
The most commonly used CPT code is 92273, electroretinography (ERG) with interpretation and report; and includes ffERG, Flash ERG and Ganzfield ERG.**
** Billing and Coding: Electroretinography (ERG) (A57677). Accessed April 1, 2023. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleId=57677&ver=5
Benefits of RETeval device ERG
Objective: Patients cannot influence results; neither can eye opacities.
Predictive: ERG tests have a high predictive value as a tool for diagnostic purposes.
Functional Data: Functional data combined with structural analysis provides a more comprehensive picture with which to make clinical decisions.
No Corneal Contact: The non-invasive, patented, sensor strip electrodes mean no corneal electrode required.
Simple to Interpret: Color-coded results allow for easy interpretation. Test results are shown on the device or it can be exported to PDF for easy integration into medical records.
Advanced Testing: Advanced full-field ganzfeld functionality in a hand-held ERG/VEP device.
Dilation Not Required: Real-time pupillography adjusts for pupil size in real time—it can be used with any pupil size.
Simple to Bill: Reimbursed and co-billable. Can be billed on the same day with OCT, photos, and or visual field testing.
The RETeval device impacts bottom lines in more ways than one. In addition to straightforward reimbursement, the test elevates practice efficiency and can boost patient loyalty. For example, because it’s handheld, it does not need a special room, and can be performed by a technician anywhere in the practice, including the waiting room.
“From the time I decide I’m going to perform an ERG test with the RETeval device, the entire process, from creating a patient profile to finishing the test, takes only minutes,” said Dr. Gerson. “And I can delegate that to a staff member if I’m busy. This can really alleviate bottlenecks in busy practices. Another benefit is that if a patient is already coming in for fundus photos or OCT analysis, you can add the RETeval test without creating a separate appointment.”
The RETeval device also can add to practice efficiency by streamlining treatment planning. For example, Dr. Cymbor notes: “When I’m looking at diabetic retinopathy and observing structure, I grade as best as I can by mild, moderate, and severe. With RETeval, if the DR Score is less than 21, I’m not too concerned because this score shows decent function. If the score is 21 to 23.5, that’s a concerning range, and I watch those patients closely. Once they hit the 23.5 cutoff, then I look at structure under a microscope and either refer or get the patient used to the idea of a referral in the near future.”
Another way that the RETeval device can elevate your practice is through patient education and strengthening doctor-patient relationships.
“The RETeval gives you a number,” said Dr. Cymbor, “And that’s a scale that virtually all patients can understand, maybe even more than a structural image or photo, because patients aren’t optometrists. A number is super easy to explain and it helps patients see that they have a goal to work towards, and they can count on me to help them realize that goal. Ultimately, the RETeval device gives me peace of mind. I know that if I have a number below 23.5 I usually don’t have to refer out—and I eliminate the uncertainty I used to have about that.”
A call to action: diabetic retinopathy fundamentals
“There is a dire need for an elevated protocol to guide patient care in diabetic retinopathy,” said Dr. Gerson. “The sad reality is that the complexity of this disease and the subjectivity of many of our tests can lead to errors and vision loss.” This sentiment led to the formation of a DR task force, comprised of medical optometry educators who aimed to simplify and standardize DR assessment and management. At the group’s inaugural meeting, held in 2022, every member of the task force agreed that structure, although important, isn’t enough to comprehensively monitor patients who have diabetic eye disease. Rather, to properly stage diabetic retinopathy (DR) and to safely assess risk of progression, both structural and functional measures are needed. Furthermore, the group concluded, subjective findings such as visual acuity are a poor surrogate for objective functional tests, such as ERG.
“Our goal was to elevate the standard of care with proper management and vigilance, even given the challenges posed by today’s busy healthcare environments,” said Dr. Chous who led the task force.
To that end, the group recently released a consensus document, called
Modern Fundamentals of Diabetic Retinopathy Management in Optometry, in which they present a framework intended to radically simplify diabetic retinopathy management so that optometrists can confidently care for the growing population of patients with diabetes. The guidelines are based on five pillars, all of which are backed by evidence-based science. Briefly, the five pillars are as follows:
1. DETECT: Approach diabetic retinopathy as a chronic progressive disease. Being a chronic progressive disease implies that you can detect it before it advances to a more critical state. This can be achieved using both structural and functional testing.
2. GRADE: Grade diabetic retinopathy at the time of diagnosis and at each subsequent visit. Chart structural retinal damage and quantify retinal cell function.
3. ASSESS RISK: To assess risk of progression, monitor diabetic retinopathy patients over time using both structural and objective functional measures.
4. MANAGE: Utilize multi-disciplinary resources to manage all diabetic retinopathy patients, regardless of disease severity.
5. SUPPORT: Provide comprehensive patient education and strategies to help prevent disease progression.
Expanded details on each of the pillars is available at
lkc.com. Over the coming months, the task force also will be providing additional in-depth explanations and responding to FAQs in optometry’s print publications and at local, national and virtual meetings.
To learn more about resources associated with these new guidelines, visit LKC.com.