Pentosan polysulfate sodium (PPS) (Elmiron; Janssen Pharmaceuticals) received Federal Drug Administration (FDA) approval for the treatment of interstitial cystitis (IC) in 1996. It is a glycosaminoglycan-like macromolecule and has become a mainstay in the treatment of IC. The exact mechanism of its action for treating IC was not clear, although it is thought that PPS coats the bladder epithelium to protect it from potential irritants.1
Recently, reports have surfaced of maculopathy secondary to PPS;2,3 however, large database analysis has confounded a clear association of macular toxicity from PPS use.4 Here, we describe a case of maculopathy secondary to PPS mimicking pattern dystrophy.
Case
A 63-year-old myopic female presented with a complaint of vision loss and metamorphopsia OS for 1 month. Previous medical history was significant for IC, Sjogren's Syndrome, controlled type 2 diabetes, fibromyalgia, and osteoarthritis. The patient’s IC had been treated with PPS 100 mg oral, three times daily, for the preceding 20 years.
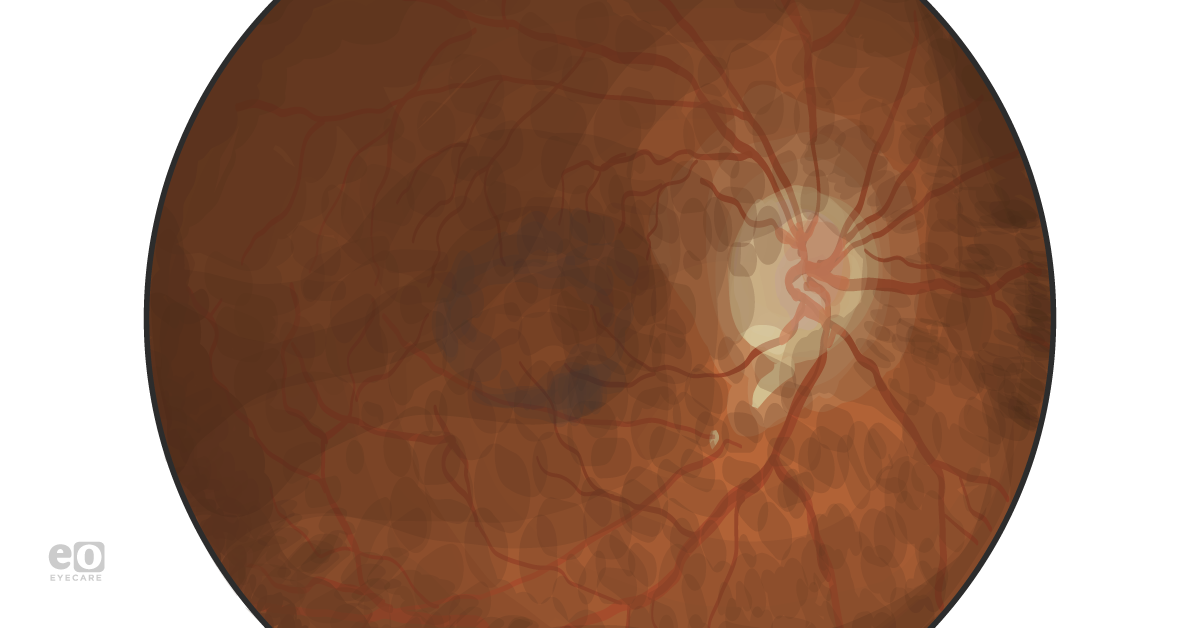
Anterior segment examination was unremarkable other than trace nuclear sclerosis OU. On posterior segment examination and color fundus photography, patients showed lacquer cracks and perifoveal pigment clumping OU (Figure 1).
Figure 1.
Optical coherence tomography (OCT) demonstrated small pseudo-vitelliform lesions at the level of the retinal pigment epithelium that corresponded to the hyperpigmented macular areas (Figure 2, arrows).
Figure 2.
Fluorescein angiography (FA) demonstrated a densely packed pattern of hypo- and hyperfluorescence in the macula OD > OS (Figure 3).
Figure 3.
Given the causal link between PPS use and maculopathy, and given the possible comorbid myopic phenotype, the patient was counseled to stop PPS use.
Discussion
IC is a chronic and incurable condition causing bladder pressure, bladder pain, or sometimes pelvic pain.5 It usually affects women and can have a negative impact on quality of life. IC is thought to relate to aberrant innervation of the bladder that results in a smaller threshold for contraction and voiding.5 PPS has been used to treat IC without safety concerns since its FDA approval in 1996. Our case demonstrates macular toxicity induced by PPS in a patient with long-term use of the medication.
In 2018, the first case series of macular toxicity associated with long-term usage of PPS were reported. It was suggested that pigmentary maculopathy was found in 6 patients undergoing long-term therapy with PPS. None of those patients had a family history of retinal disease and no pathogenic process was identified with rigorous diagnostic imaging and DNA testing.2 It shows that the dosage of PPS used among those patients ranging from 200 to 400 mg/day with a median duration of 15.5 years (range from 12 to 20).2 The most common complaint was difficulty with near vision.2 On examination, almost all affected eyes showed subtle paracentral hyperpigmentation at the level of retinal pigment epithelium (RPE) with a surrounding array of vitelliform-like deposits. 2 Multimodal retinal imaging showed RPE abnormality, which is generally contained in a well-delineated area in the posterior pole.2
Researchers at Kaiser Permanente (Oakland, CA) conducted a clinical study of 140 patients who had taken PPS for a minimum of 5 years.
4 Among those patients, 91 agreed to an eye examination. Twenty-two (24%) of the 91 patients showed signs of drug toxicity.
4 In addition, the rate of toxicity rose with the amount of PPS consumed, from 11 percent of those taking 500 to 1,000 grams to 42 percent of those taking 1,500 grams or more.
4 Those results might suggest that PPS associated retinal toxicity is related to the drug accumulation in the retina over time.
Further study is required to confirm this.
Jain et al. used a large national cohort to determine if there is an association between PPS use and macular disease.
6 In this study, based on the data from a US medical claims database from 2002 to 2016, a total of 3012 and 1604 PPS users were compared with 15,060 and 8,017 matched controls at 5 and 7 years, respectively.
6 The primary outcome measures in this study included: (1) any new diagnosis of a hereditary or secondary pigmentary maculopathy (atypical maculopathy outcome), and (2) any new diagnosis of dry
age-related macular degeneration (AMD). Multivariate analysis showed no significant association at 5 years (p>0.13); however, at 7 years, PPS users had significantly increased odds of having the atypical maculopathy and AMD (OR=1.41, 95% CI 1.09 to 1.83, p=0.009).
6Our case demonstrates similar paracentral RPE hyperpigmentation with vitelliform-like deposits to those maculopathy cases recently published and can resemble pattern dystrophy.2 Moreover, the duration of PPS exposure is congruent with toxicity profiles seen in treated IC populations. Finally, the presence of myopia in our patient leads us to conclude that—similar to other drugs capable of toxic maculopathy (e.g., hydroxychloroquine)—the concomitant presence of comorbid macular disease may warrant close monitoring or early cessation of PPS use in hopes of preventing macular changes and possible vision decline.
Conclusions
There is increasing recent evidence of macular toxicity induced by PPS in the literature. This raises serious concerns of progressive maculopathy over time. In addition, patients with a comorbid disease (myopic degeneration, age-related macular degeneration) may be more susceptible to macular changes. Consequently, clinicians should be aware of this condition as it may be mistaken for other well-known macular disorders, such as pattern dystrophy. Pattern dystrophies are a group of autosomal dominant macular diseases in which accumulation of lipofuscin causes damage to the retina tissue. They are characterized by various manifestations of pigment deposits in the macula, including egg yolks, butterflies, or knotted fishnet patterns.
Although there is no ophthalmic screening guideline for PPS use currently, eye care providers should promptly identify this vision-threatening condition at an early stage among affected patients with serial examinations and imaging to prevent progressive vision loss. Further studies are required to help identify the safe dosage and duration of PPS use, as well as the screening frequency and optimal early diagnostic imaging modalities.
References:
- Nickel JC, Moldwin R. FDA BRUDAC 2018 Criteria for Interstitial Cystitis/Bladder Pain Syndrome Clinical Trials: Future Direction for Research. J Urol 2018; 200:39-42.
- Pearce WA, Hanif AM, Jain N. Re: FDA BRUDAC 2018 Criteria for Interstitial Cystitis/Bladder Pain Syndrome Clinical Trials: Future Direction for Research: J. C. Nickel and R. Moldwin J Urol 2018;200:39-42. J Urol 2018;200:1122-1123.
- Huckfeldt R, et al. Progressive Maculopathy After Discontinuation of Pentosan Polysulfate Sodium. Ophthalmic Surgery, Lasers & Imaging Retina 2019; 50:656-659.
- American Academy of Ophthalmology News Releases. More Evidence Linking Common Bladder Medication to a Vision-threatening Eye Condition. October 12, 2019. Accessed December 15, 2019 at https://www.aao.org/newsroom/news-releases/detail/evidence-linking-bladder-medication-eye-condition.
- Interstitial Cystitis. Mayo Clinic. www.mayoclinic.org/diseases-conditions/interstitial-cystitis/symptoms-causes/syc-20354357. Accessed January 15, 2020.
- Jain N, Li AL, Yu Y, VanderBeek BL. Association of macular disease with long-term use of pentosan polysulfate sodium: findings from a US cohort. Br J Ophthalmol 2019. Nov 6. pii: bjophthalmol-2019-314765. doi: 10.1136/bjophthalmol-2019-314765.