First described by physiologist François Magendie as “neuroparalytic keratitis” in 1824,
neurotrophic keratitis (NK) is a degenerative corneal disease caused by disruption to trigeminal sensory innervation of the tissue.
1-4This damage to the corneal nerves or associated trigeminal sensory pathway results in diminished or absent corneal sensation, disrupted epithelial cell turnover, impaired wound healing, and abnormal blinking and tear production.3-12
These effects can disrupt normal corneal and ocular surface homeostasis, resulting in a spontaneous breakdown of the corneal surface that could ultimately lead to vision-threatening scarring and even corneal perforation.2,3,6,13
Though often viewed as a particularly difficult condition to manage, there have been numerous advancements that have greatly improved eyecare providers' (ECPs) ability to treat this potentially debilitating disease.
Although NK has traditionally been considered a rare disease with an estimated 65,000 to 70,000 cases in the US, it is likely this is a gross underestimation.4,14 One of the many reasons for this misconception is that NK can be easily missed or misattributed to other more common ocular surface conditions.
This includes
dry eye, persistent corneal epithelial defects (PCED), and anterior basement dystrophy (ABMD), to name a few—especially in their early stages, given each has relatively nonspecific symptoms. This makes early detection, diagnosis, and proactive treatment of NK a key to successfully managing the disease.
Anatomy and physiology of the corneal nerves
The cornea is the most heavily innervated tissue in the body, with 7,000 nerve endings per square millimeter.
3,5 The reason for such heavy innervation is likely due to the vital role the corneal nerves play in corneal and
ocular surface homeostasis.
The corneal epithelial cells and nerves have a reciprocal relationship, with each providing trophic support to the other. The cornea is by necessity avascular to maintain optical clarity and, therefore, receives nourishment from the tear film, aqueous humor, and from the corneal nerves in the form of neuropeptides and neurotransmitters.5,6
These trophic factors allow for routine epithelial cell turnover, cellular maintenance, and wound healing.3,5-7,13,15 In return, the corneal epithelial cells provide neurotrophins, such as nerve growth factor (NGF), that support nerve maintenance, growth, and repair.5,15
Nerve damage disrupts ocular surface homeostasis
Additionally, the corneal nerves function as a vital component of the trigeminal reflex arc responsible for normal blinking and tear secretion via sensory feedback.4,7,8,11,13
Damage to the corneal nerve directly, or at any point along the trigeminal sensory pathway from which these nerves originate, fundamentally disrupts the delicate interplay between them and corneal epithelial cells owing to a loss of trophic support to the epithelial cells and vice versa.
Further, the absence or diminished corneal sensation can interfere with the normal blink reflex and invariably tear production. The result tends to be a breakdown of the corneal surface due to the disruption to normal epithelial cell turnover, impaired corneal wound healing, and tear film and ocular surface instability.3,4,6-8,11,13,16
Stages of neurotrophic keratitis
The subsequent breakdown of the corneal surface is traditionally categorized into three stages based on the Mackie Classification System:2-4,6-8,13,17,18
Stage 1 neurotrophic keratitis
Early NK is characterized by relatively subtle corneal epithelium changes and nonspecific symptoms. These epithelial changes include a dull or cloudy corneal appearance, superficial punctate keratitis, or an area of focal dried epithelium.
Conjunctival staining and tear film instability (i.e., decreased tear break-up time) can also be present. These NK patients might present similarly to those with dry eye, including complaints of general irritation and blurred vision. In chronic cases, corneal epithelial hyperplasia and irregularities may present along with neovascularization and stromal scarring.
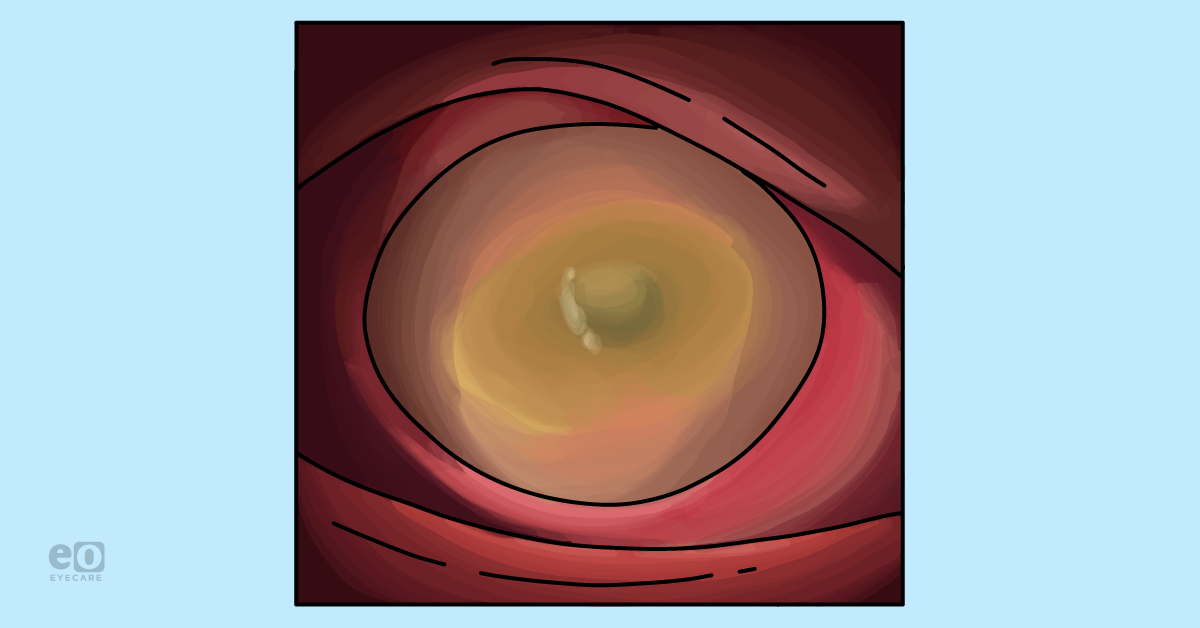
Figure 1 highlights a clinical image of a patient with Stage 1 neurotrophic keratitis.
Figure 1: Courtesy of Cory J. Lappin, OD, MS, FAAO.
Stage 2 neurotrophic keratitis
Once NK has reached a moderate level, the cornea degenerates further, as evidenced by the appearance of persistent epithelial defects (PEDs). PEDs are non-healing disruptions to the corneal epithelium with smooth, rolled edges consisting of edematous, loosely adherent epithelial cells and are typically circular or oval in shape. Descemet’s folds can also appear at this stage due to corneal edema.
Figure 2 shows a patient with Stage 2 neurotrophic keratitis.
Figure 2: Courtesy of Cory J. Lappin, OD, MS, FAAO.
Stage 3 neurotrophic keratitis
Severe NK signals a near-total breakdown of the corneal tissue in the form of
corneal ulceration with stromal involvement. Patients at this stage are at risk of corneal melting and perforation. Similar to PEDs, these ulcers usually illustrate smooth, rolled edges. Though rare, a sterile hypopyon may develop as well due to the inflammatory response within the anterior chamber.
Figure 3 demonstrates a patient with Stage 3 neurotrophic keratitis.
Figure 3: Courtesy of Cory J. Lappin, OD, MS, FAAO.
Comparing the three stages of NK
Although NK presents differently depending on its severity, the commonality shared by all stages of the disease is a reduction in, or complete absence of, corneal sensation.2-4,6-8,13,19,20 This tends to be the reason why symptoms at Stages 2 and 3 can vary from complaints of pain and irritation to being completely asymptomatic despite the presence of significant corneal defects.
This potential disconnect between signs and symptoms is caused by the underlying pathophysiology of NK—damage to the corneal nerves (specifically to the sub-basal plexus) resulting in corneal hypoesthesia or anesthesia. So, not only does the loss of nerve function cause a breakdown of the cornea, but it also impairs the patient’s ability to feel the impact of these pathophysiological changes.
Therefore, the damage to the corneal surface that would normally cause a significant pain reflex now causes little to no discomfort because these nerves are no longer able to effectively transmit signals at an amplitude for the brain to interpret this sensation.
The characteristic alteration in corneal sensation is consequently the key to diagnosing the disease.
Diagnosing neurotrophic keratitis
The
pathognomonic sign of NK is reduced or absent corneal sensitivity.
3,4,6-8,13,19,20 As was discussed previously, damage to the corneal nerves can be considered the root cause of NK, resulting in impairment of corneal sensation as hypoesthesia or anesthesia.
Diagnostically, corneal sensitivity can be assessed in several different ways. Quantitatively, a Cochet-Bonnet aesthesiometer can be used to measure the degree of corneal sensitivity impairment.3,6,21 However, a Cochet-Bonnet aesthesiometer is often primarily used in academic settings, and many practicing ECPs may lack access to the device.
Performing corneal sensitivity testing to diagnose NK
Fortunately, corneal sensitivity can be quickly and easily assessed using several items commonly found in any practice setting. Corneal sensation can be qualitatively assessed by touching the corneal surface with the end of a cotton-tipped applicator teased into a wisp, the end of a tissue, or a piece of dental floss and evaluating the patient’s response.3,6,22
If the patient reports feeling a reduced or absent sensation of touch when the end of the chosen implement makes contact with the corneal surface, this is indicative of impaired nerve function and, consequently, NK.
When performing corneal sensitivity testing, it is strongly recommended to test all four quadrants of the cornea in addition to centrally, regardless of instrument. The reason for this is that nerve damage and resultant impairment in corneal sensation can be isolated to one area of the cornea rather than present over the entire surface.19
Checking corneal sensitivity in all four quadrants
For example, if corneal sensitivity is only assessed centrally while a patient has nerve damage and anesthesia that is localized to the superior quadrant, the patient will display seemingly normal corneal sensitivity despite truly having NK.19
These potential regional differences in corneal sensation underscore another important but often misunderstood concept regarding the nature of NK, which is the possibility that a patient may have a neurotrophic cornea while still experiencing pain and discomfort.
For instance, a patient that has absent corneal sensation that is limited to only one quadrant might display significant discomfort owing to the fact that the corneal nerves in the rest of the tissue are still functional and capable of transmitting pain signals associated with any corneal defects present in these areas.19
This is the reason the phrase “stain without pain,” which is often associated with NK, can be used as a helpful guidepost but is not a statement of diagnostic necessity.3,4,6,19 If a patient does present with signs of corneal disruption out of proportion to the pain that is reported, then NK should be immediately suspected.
However, this is not always the case, especially in the early stages of the disease. Thus, the observation of reduced or absent corneal sensation can allow for a diagnosis of NK, even if the patient reports some degree of discomfort or irritation.
NK signs and symptoms to look for in a case history
Additionally, a thorough case history is imperative to potentially uncovering the disease if a patient has a condition that might predispose them to the development of NK.3,4,6-8,13,19,20
It is again worth noting that many of the signs and symptoms of NK are nonspecific and mimic those seen in more common ocular surface diseases, such as dry eye, PCED, or ABMD, especially in the early stages of the disease.
In vivo corneal confocal microscopy can be utilized to allow for noninvasive visualization of the corneal nerves, which can display a reduction in sub-basal nerve plexus density in cases of NK; however, while helpful information, this is not required to diagnose the disease.
7 Understanding the many causes of NK
While the ultimate presentation of NK is universal, there are a myriad of causes of NK. All these differing etiologies share one commonality: they may cause various levels of damage to the corneal nerves or the trigeminal sensory pathway from which they arise.
Neurotrophic keratitis can be caused by:
- Infection
- Trauma
- Surgery
- Systemic disease
- Chronic ocular surface disease (OSD)
- Compressive lesions
- Genetic and congenital diseases
- Iatrogenic damage
NK caused by herpetic infection and refractive surgery
Two commonly encountered causes of NK are herpetic infection and refractive surgery. Herpes viruses, including
simplex and
zoster, can lay dormant in the trigeminal ganglion and then reactivate, which may result in an inflammatory response with resultant damage to the nerves.
23-25In cases of
refractive surgery, such as laser-assisted in situ keratomileusis (LASIK) or photorefractive keratectomy (PRK), the corneal nerves are severed during flap creation or the ablation process itself.
26The use of panretinal photocoagulation (PRP) to treat retinal disorders and
retinal detachment repair can also damage the long ciliary nerves, having a reciprocal impact on the ocular surface as persistent corneal erosions which may mask the earlier stages of NK.
27-31Non-ocular causes of NK
Non-ocular surgeries can also result in NK, such as tumor removal or ablative procedures for trigeminal neuralgia.32 Likewise, tumors can act as compressive lesions, as can aneurysms, which cause mechanical nerve damage.7,8,33-37
In terms of systemic disease,
diabetes can induce NK due to microvascular damage to the blood vessels that supply the nerves with oxygen and nutrients, which is the same mechanism underlying the foot ulcers often observed in diabetes.
29,38-40NK caused by chronic OSD and topical ophthalmic medications
Chronic ocular surface disease, such as uncontrolled dry eye or PCED, can lead to persistent tear film instability and ocular surface inflammation, which may induce nerve damage.17,20,41-43 Physical, chemical, or thermal injury to the cornea can directly cause nerve damage.3,4,7,44,45
NK can also be a complication of chronic use of
topical ophthalmic medications often seen in glaucoma patients who require polytherapy several times a day to adequately manage their intraocular pressure but inadvertently suffer from corneal toxicity due to preservation system, including benzalkonium chloride (BAK).
46Additionally, topical beta blockers such as timolol and betaxolol have been specifically associated with an increased risk of NK, as has the use of topical non-steroidal anti-inflammatory drugs (NSAIDs) and anesthetics, since these drops can disrupt nerve function.44,47,48
NK caused by contact lens wear and congenital diseases
Contact lens wearers, especially those who
misuse their lenses, are also at risk for developing nerve damage.
44There are numerous congenital and genetic diseases that cause abnormal nerve function, such as:49-55
- Congenital corneal hypoesthesia
- Riley-Day syndrome
- Goldenhar-Gorlin syndrome
- Moebius syndrome
- Familial corneal hypoesthesia
- Congenital insensitivity to pain with anhidrosis
- Gómez-López-Hernández syndrome
Advancements in NK treatment
There are three primary goals of NK treatment:
- Stabilize the corneal surface.
- Prevent progression.
- Repair the nerve damage underlying the disease.
The range of NK treatments spans the use of
artificial tears to surgical procedures and nerve transplantation. Although certain treatments have been traditionally recommended for each stage of NK, these guidelines are not axiomatic due to the fact that, in reality, the progression of NK exists on a continuum.
Therefore, treatments typically reserved for later stages may not only be beneficial in the treatment of early NK, but may also be a better overall option than what is typically recommended.
Likewise,
NK should ideally be diagnosed and treated at the earliest stage possible to prevent the development of vision and eye-threatening ulcers,
scarring, and perforation, even though traditionally, the emphasis of NK detection and intervention has primarily focused on more advanced stages of the disease.
The treatments for NK, as recommended by the disease stage, include the following:3,4,6-8,13,17-20,56
Stage 1 NK treatments
Therapies traditionally implemented when treating early NK include preservative-free lubricating drops, gels, and ointments, as well as the use of biologic treatments, including
autologous serum tears, platelet-rich plasma, and umbilical cord serum.
The aims of these biologic treatments are improving tear film stability, repairing epithelial damage, and encouraging nerve repair through their anti-inflammatory and regenerative properties stemming from the trophic factors they contain within.57-63
Stage 2 NK treatments
In moderate stages, low-dose tetracycline or macrolide antibiotics (such as doxycycline or azithromycin) can be prescribed for their anti-inflammatory and matrix metalloproteinase-inhibiting properties, which might help prevent the physical breakdown of the corneal tissue.64
Lid taping and the use of soft bandage contact lenses (with prophylactic antibiotic coverage strongly advised) can be helpful in stabilizing the corneal surface, especially in the presence of PCEDs. At this stage, the use of cryopreserved amniotic membranes and topical NGF such as cenegermin may be recommended.
These two biologic treatments have similar functions.
Cryopreserved amniotic membranes have anti-inflammatory, anti-angiogenic, and anti-fibrotic properties due to the presence of trophic and growth factors, especially NGF.
65-71The role of NGF in treating neurotrophic keratitis
Additionally, topical NGF itself is capable of repairing damaged nerves.6,72 Topical insulin has also been used as it may encourage epithelial cell migration as well as corneal wound healing and nerve repair.73,74
NGF is unique in its ability to address the underlying cause of NK by repairing nerve damage, which in turn can restore corneal sensation, promote epithelial cell proliferation, encourage corneal wound healing, and improve blinking and tear secretion, which are reliant on sensory feedback.4,6,18,72,75-82
Mechanistically, NGF binds to high-affinity TrkA and low-affinity p75NTR NGF receptors that are present throughout the ocular surface, including all layers of the corneal epithelium, the lacrimal gland, and sensory nerves.5,76,82,83
Therefore, its direct effects on the corneal nerves, epithelial wound healing, and tear production have made NGF a particularly effective treatment option at all stages of the disease.84-88
Stage 3 NK treatments
In severe NK, at which point corneal ulceration with stromal involvement raises the risk for corneal melting and perforation, surgical intervention is the mainstay of treatment.
Tarsorrhaphy, or the creation of a conjunctival flap, can stabilize the corneal surface even in advanced cases of corneal degeneration.
4,8,19,20However, while the use of tarsorrhaphy or conjunctival flaps can stabilize the surface and prevent progression, these procedures tend to result in poor visual function and concerns surrounding cosmesis.3,89,90 Therefore, they can be less than desirable long-term treatment options.
Alternatively, corneal neurotization surgery aims to restore corneal nerve function by transplanting a healthy autologous or allogenic nerve tissue donor to the site of damage to possibly return function to the cornea.7,17,19,20,56,91
The use of a keratoprosthesis has also been demonstrated as an effective treatment for late-stage NK.
92 Additionally, the use of cyanoacrylate or fibrin glue can be used to address
corneal perforations.
Treatments to consider with caution for NK
Three treatments that should be avoided or used with extreme caution are topical corticosteroids,
NSAIDs, and
corneal transplantation surgery. When the corneal epithelium tissue has been compromised, both topical steroids and NSAIDs can carry an increased risk of corneal melting and perforation.
3,4,7,93,94Keratoplasty in patients with NK can unfortunately translate to less than favorable prognosis since the donor tissue transplantation does not address the underlying nerve damage causing the disease.
Further, the donor corneal tissue will contain nerves that have been severed during the transplantation process, which in turn often subjects the graft to the same neurotrophic complications as the patient’s own cornea.4,7,8,95,96
Neurotrophic keratitis treatment summary
When choosing which treatments to implement in the
management of NK, it is helpful to recall the three aims of NK treatments.
Although stabilization and prevention of progression are indeed important aspects of managing NK, many of the therapies that achieve this stabilization do not inherently address the underlying damage to the trigeminal sensory pathway or corneal nerves. Therefore, many advancements in the treatment of NK have centered on repairing and healing the nerve damage at the root of the disease.
For this reason, biologics treatments, including autologous serum tears,
platelet-rich plasma, umbilical cord serum, topical insulin, cryopreserved amniotic membranes, and topical NGF such as cenegermin, confer a significant advantage in treating NK as they can not only stabilize the corneal surface but encourage healing and restoration of nerve function.
In terms of surgical procedures, corneal neurotization may restore nerve function compared to tarsorrhaphy and conjunctival flap creation, which stabilize the condition but do not directly treat the underlying nerve damage. However, corneal neurotization surgery is a highly specific and technical procedure, so a patient’s access to a surgeon capable of performing the surgery may be limited.
Managing concomitant OSD in NK patients
Additionally, it is strongly recommended to manage any concomitant ocular surface (i.e.,
dry eye, meibomian gland dysfunction [
MGD],
blepharitis) or glaucomatous disease. Not only will treating these conditions help create an ocular surface environment that is conducive to healing by improving tear film stability and reducing inflammation, but it will also potentially alleviate one of the contributing factors responsible for a patient developing NK in the first place.
Some basic, foundational dry eye and general ocular surface treatments include
omega-3 fatty acid supplementation (which may also encourage nerve repair),
97 blink exercises, lid hygiene, warm compresses, and preservative-free artificial tears. If a patient has a component of corneal exposure, the use of moisture chamber goggles during sleep can be helpful, as can scleral lens wear during the day.
Turning to
glaucoma management, treatment consideration may be given to switching from preserved to preservative-free medications based on disease severity. For patients in need of chronic inflammatory control, the use of topical immunomodulators, such as cyclosporine and lifitegrast, can be beneficial. In cases of aqueous deficiency, neurostimulatory agents like varenicline can be utilized. Likewise, tear film stabilizers, such as perfluorohexyloctane, can help manage the evaporative element of dry eye.
Advanced procedures, including
intense pulsed light (IPL),
radiofrequency (RF), and thermal pulsation/expression, are also highly effective means of addressing dry eye and various ocular surface diseases. Microblepharoexfoliation can be helpful in managing concomitant blepharitis, as can the use of topical lotilaner for cases of
Demodex blepharitis.
Final NK words of wisdom
NK is a chronic and progressive condition caused by damage to the nerves that supply the cornea with sensory innervation and will, therefore, advance without intervention. Traditionally, the emphasis of NK has focused on Stages 2 and 3, at which point the cornea has already experienced relatively significant damage.
For this reason, many of the treatments classically used in NK were aimed at stabilizing the condition and preventing progression rather than directly addressing the nerve damage underlying the disease. However, recent advancements in treatment have placed a greater emphasis on early detection and proactive treatment of NK, specifically when cornea disruption is often mild and subtle.
Therefore, the goal should be to initiate treatment earlier in the disease process before it advances to potentially vision-threatening scarring or even eye-threatening corneal perforation.