The vitreous is a gelatinous structure composed of 98% water and 2% macromolecules such as collagen.1
The vitreous, on average, accounts for 4mL of volume in the adult human eye,1 and while it is the largest ocular structure by volume, it has gone overlooked for years due to the difficulty of imaging its interactions with the retinal surface.
Overview of the vitreous
The body of the vitreous gel is encapsulated in a cortex (posterior vitreous cortex) that is adhered to the internal limiting membrane (ILM) of the retina at birth.
As we age, two changes occur:
- The vitreous body liquefies.
- There is a breakdown of the extracellular matrix that adheres to the posterior vitreous cortex to the ILM.
As these processes occur, the vitreous body collapses, and the posterior vitreous cortex peels itself away from the ILM. This may occur in a seamless manner without negative consequences to the retina, but if tight adhesions to the retinal surface persist as the vitreous body liquefies and collapses, areas of traction can occur in both the macular region and the peripheral retina.1
This text will focus on anomalous interactions between the vitreous and the macular surface.
Anomalous interactions between the vitreous and the macular surface
This anomalous interaction results in a spectrum of macular pathologies that includes vitreomacular adhesion (VMA),
vitreomacular traction (VMT), full-thickness macular hole (FTMH), epiretinal membrane (ERM), lamellar macular hole (LMH), and macular pseudohole.
1A firm understanding of vitreomacular interface (VMI) disease and the terminology used to describe this pathological spectrum is important in making sound decisions regarding the management of affected patients.
📝
Macular Hole Staging Cheat Sheet
Use this cheat sheet as a step-by-step guide to accurately identify and classify macular holes.
Risk factors for VMI disease
Since vitreomacular interface disease is caused by age-related changes to the vitreous and VMI, it makes sense that these conditions are seen more commonly with aging. For example, only 3% of primary FTMH are seen in patients younger than 55.2 Patients of any race or sex can present with VMI disease, but there is a female predilection. It has been considered that declines in estrogen levels during menopause can cause earlier vitreous liquefication.3
Note that 72% of FTMH are reported in female patients.2 In addition, patients with high myopia or any retinal condition that alters the vitreomacular interface, including diabetes, retinal vein occlusions, and posterior segment inflammation, are at higher risk.1,2
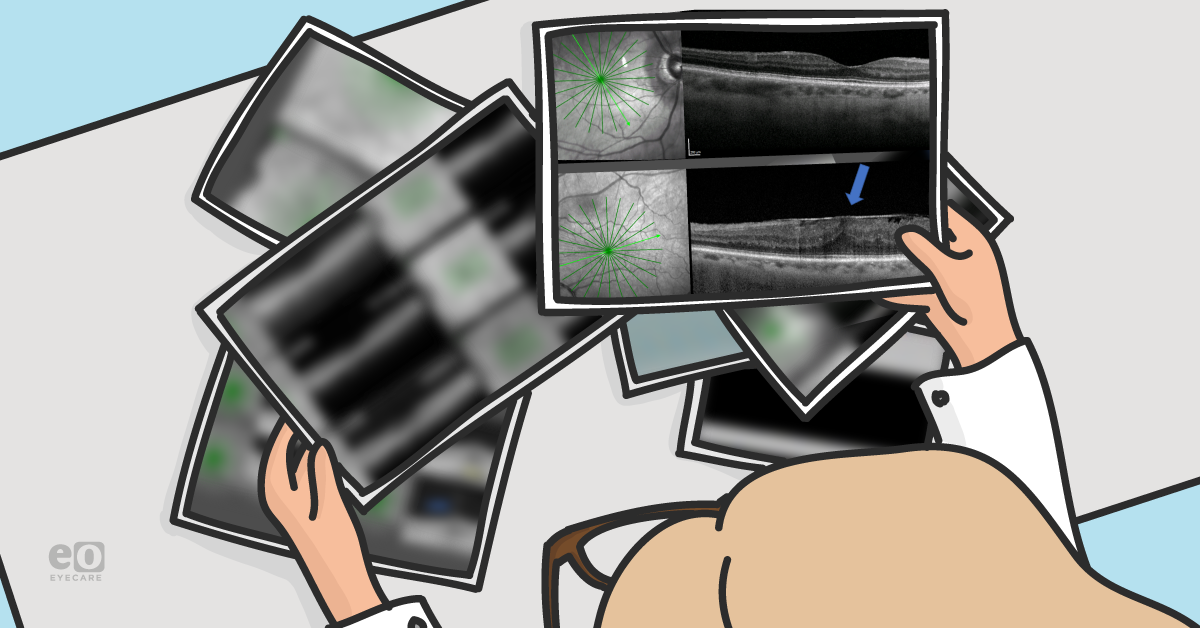
Patients who present with full-thickness macular hole in one eye have a 10 to 15% chance of developing it in the fellow eye within 5 years.4 Careful OCT evaluation of fellow eyes is required to assess the status of the posterior vitreous cortex to determine risk. If the posterior vitreous cortex is completely separated from the macular surface in the fellow eye, the risk of macular hole formation is extremely low.5
Figure 1 reveals the OD (top) and OS (bottom) of a 57-year-old female with large FTMH OD. The OS has a complete separation of the posterior vitreous cortex from the macular surface (blue arrow), making it very unlikely for the OS to develop FTMH.
Figure 1: Courtesy of Jessica Haynes, OD
Historical classification of macular holes
Macular holes were originally classified in 1988 by Gass, as seen in Table 1 below.6 This classification scheme has been widely used and adapted by numerous sources.
Even the most recent
American Academy of Ophthalmology preferred practice pattern for macular holes using this staging method when discussing recommended treatment and management options for macular holes.
4Table 1 outlines the clinical characteristics and anatomical interpretation for the Gass staging of macular holes.
| Gass Staging | Clinical Characteristics | Anatomical Interpretation |
|---|
| Stage 1A | Central yellow spot, loss of foveolar depression, no vitreofoveolar separation. | Early serous detachment of the foveolar region. |
| Stage 1B | Yellow ring with bridging interface, loss of foveolar depression, no vitreofoveolar separation. | Serous foveolar detachment with lateral displacement of xanthophyll in small rings. For larger rings, central occult, foveolar hole with centrifugal displacement of foveolar retina and xanthyphyll with bridging contracted prefoveolar vitreous cortex. |
| Stage 2 | Eccentric oval, crescent, or horseshoe retinal defect inside edge of yellow ring, central round retinal defect with rim of elevated retina. | Hole in contracted prefoveolar vitreous bridging round retinal hole, no loss of foveolar retina. Hole with pseudo-operculum, rim of retinal detachment. |
| Stage 3 | Central round defect ≥400μm in diameter, no Weiss ring, rim of elevated retina. | Hole with pseudo-operculum, no posterior vitreous detachment. |
| Stage 4 | Central round retinal defect, rim of elevated retina, Weiss ring present. | Hole with pseudo-opercula and posterior vitreous detachment from optic disc and macula. |
However, it is important to note that this classification scheme was developed prior to the development and use of OCT in eyecare. This staging system is based on what can be visualized with careful fundus examination at the slit lamp. Macular hole terminology became disjointed with the attempt to integrate OCT findings with this widely used fundoscopic classification scheme.
Table 2 highlights the IVTS classifications for macular holes based on OCT findings.
| Anatomic State | IVTS Classification Based On OCT |
|---|
| Vitreomacular adhesion | Lifting of the perifoveal vitreous cortex from the macular with residual attachments with a 3mm radius of the fovea, no detectable change in foveal contour or underlying retinal tissue, focal ≤1500μm, broad >1500μm, and concurrent or isolated based on the presence or absence of concurrent macular disease. |
| Vitreomacular traction | Lifting of the perifoveal vitreous cortex from the macular with residual attachments with a 3mm radius of the fovea, associated distortion of the foveal surface, intraretinal structural changes, and/or elevation of the fovea above the retinal pigment epithelium (RPE), but with no full-thickness interruption of all retinal layers, focal ≤1500μm, broad >1500μm, and concurrent or isolated based on the presence or absence of concurrent macular disease. |
| Full-thickness macular hole | Full-thickness foveal lesion that interrupts all macular neural layers from the ILM to the RPE (RPE remains intact), categorized by the presence or absence of VMT, categorized by cause: primary (caused by VMT) or secondary (i.e., trauma or other cause), small (≤250μm), medium (>250μm and ≤400μm), or large (>400μm). |
Despite best efforts, terminology remains somewhat inconsistent and disputed, creating confusion for providers who manage or co-manage patients with VMI disease. This article aims to review OCT-based terminologies and discuss best practice patterns for patients with VMI disease.
Vitreomacular adhesion (VMA)
VMA is an OCT-based finding defined by the IVTS as a partial separation of the posterior vitreous cortex from the macular surface with residual vitreous attachment within a 3mm radius from the fovea. To be classified as VMA, there must be no structural alteration to the retina resulting from the vitreous adhesion.
This is essentially a normal finding of age, often seen during
routine OCT imaging or when using OCT to evaluate other pathologies. Patients with VMA are typically asymptomatic. In this stage, there is a high likelihood that vitreous separation will continue without negative consequences, but anomalous interactions can occur, leading to the pathological processes outlined below. Thus, management of VMA is observation only.
Figure 2 shows the OCT imaging of a 60-year-old female patient who presented with medium-sized FTMH OD. She has successful closure with vitrectomy, ILM peeling, and fluid gas exchange.
Figure 2: Courtesy of Jessica Haynes, OD
Figure 3 features the OS presented with focal VMA (top), which progresses to focal VMT (middle) with slight disruption of the ellipsoid zone (red arrow). She then experiences PVD-type symptoms (flashes and floaters) and presents with complete separation of the posterior vitreous cortex (blue arrow) from the macular surface (bottom). The patient was encouraged that the posterior vitreous cortex has now separated without the formation of FTMH.
Figure 3: Courtesy of Jessica Haynes, OD
VMA has been further classified based on the width of its adhesion. Focal VMA has been defined as adhesions that are ≤1500μm, and broad adhesions are defined as those >1500μm. It is unclear if there are any clinical implications to focal versus broad VMA.1
VMA was also categorized by the IVTS as either concurrent or isolated. Concurrent VMA occurs in eyes with other macular pathologies such as diabetic macular edema, retinal vein occlusions, or
age-related macular degeneration (AMD). Isolated VMA occurs in otherwise normal maculas.
1Vitreomacular traction (VMT)
Vitreomacular traction is also primarily an OCT-based finding; however, patients at this stage may present asymptomatically or with visual symptoms, such as reduced visual acuity,
metamorphopsias, and scotomas.
Figure 4 demonstrates a 57-year-old female patient who presented with reduced VA (20/40) and symptoms of metamorphopsia/scotoma (top left) from a focal VMT creating inner retinal cystic spaces (red arrow) and subfoveal ellipsoid zone disruption (blue arrow) (bottom left). The patient was monitored without any intervention. She had spontaneous resolution of the VMT, normalized foveal anatomy, improved visual acuity, and near total resolution of metamorphopsias at 2 months.
Figure 4: Courtesy of Jessica Haynes, OD
The clinical examination may remain normal, but careful examination may reveal subtle findings of foveal yellowing, distorted/absent foveal light reflex, or cystoid macular appearance.
The IVTS defined VMT based on OCT findings where all of the following criteria must be met:
- Evidence of perifoveal vitreous cortex detachment from the retinal surface
- Macular attachment of the vitreous cortex within a 3mm radius of the fovea
- Association of attachment with distortion of the foveal surface, intraretinal structural changes, the elevation of the fovea above the RPE, or a combination thereof, but no full-thickness interruption of all retinal layers
In the Gass classification system, VMT would be considered stage 1A and 1B holes.
Figure 5 highlights OCT imaging of a variety of VMT presentations. A: Asymptomatic focal VMT with inner retinal cystic spaces. This patient was monitored. B: Symptomatic focal VMT with inner retinal cystic spaces, concurrent ERM, and subfoveal vitelliform lesion from chronic photoreceptor elevation. This patient had surgical intervention. C: Symptomatic focal VMT with inner retinal cystic spaces and large subfoveal outer retinal cyst. This patient had surgical intervention. D: Asymptomatic broad VMT with altered foveal contour. This patient was monitored.
Figure 5: Courtesy of Jessica Haynes, OD
Comparing broad and focal VMT
VMT, like VMA, was also classified as focal (≤1500μm) or broad (>1500μm) and isolated or concurrent by the IVTS. The distinction of focal versus broad for VMT is of clinical importance because of their tendencies to lead to different outcomes.
Focal VMT is more likely to result in the formation of macular holes, tractional foveal cysts, and foveal detachments, while broad VMT is more likely to cause diffuse macular thickening and epiretinal membranes.7 While focal VMT is more likely to create visual disturbance and macular hole formation in the short term, it is important to remember that broad VMT may lead to focal VMT.
Figure 6 shows a 60-year-old female with a history of macular hole requiring surgery OD presents with asymptomatic VMT OS that begins as broad VMT (9/21/2018). The OS is monitored without intervention until 10/4/2019, when the VMT progresses to a focal V-shaped VMT with symptoms of reduced visual acuity and metamorphopsia. Risks, benefits, and alternatives to surgery were discussed with consideration for recent progression, visual symptoms, and status of the OD (required surgery for FTMH). Surgical intervention with
vitrectomy, ILM peeling, and fluid gas exchange successfully results in 20/25 visual acuity.
Figure 6: Courtesy of Jessica Haynes, OD
Comparing V- and J-shaped VMT configurations
In addition, VMT has been classified by other sources according to its configuration as V-shaped or J-shaped.7 V-shaped VMT has lifting of the posterior vitreous cortex on both the nasal and temporal aspects of the macula. J-shaped has lifting of the posterior vitreous cortex on the temporal side with residual broad attachment on the nasal side.
In general, V-shaped VMT is most associated with focal adhesions and complications of focal adhesions, while J-shaped VMT is most associated with broad adhesions and their complications.
Bottos et al. suggest that classification based on adhesion diameter is more predictive of macular pathology than classification based on this J- or V- morphology.
9Managing and treating vitreomacular traction
The goal of the IVTS was to unify providers in the terminologies used when discussing vitreomacular interface disease; however, there still does not exist a definitive management recommendation for VMT. Factors that lead to difficulty in this area are the possibility for spontaneous resolution of VMT, various treatment methods, and a wide array of VMT presentations.
Spontaneous resolution of VMT may be as high as 50%,4 with resolution or improvement of symptoms. Additionally, the range of VMT presentations leads to varying patient symptoms, different anatomical disruptions, and different risks of long-term visual disability.
Due to the variety of treatment options available for VMT as well as the possibility for spontaneous resolution, management decisions are ultimately made based on numerous factors such as visual symptoms, the status of the fellow eye, patient’s age, health status, visual expectations, and financial limitations, as well as patient and doctor preferences.12
VMT can progress to the formation of full-thickness macular holes, as discussed below. Even without resulting in a full-thickness macular hole, traction on the fovea can result in other alterations or disruption to the foveal contour once traction is released, such as macular schisis, lamellar macular hole, or photoreceptor disruption.
Surgical treatments for VMT
Surgical options for VMT include vitrectomy alone as well as vitrectomy in combination with the peeling of epiretinal membranes, peeling of the ILM, and air or gas tamponades. There are also many reports of the successful use of pneumatic vitreolysis for symptomatic patients with focal VMT.
This involves the injection of a gas into the vitreous cavity to cleave the vitreomacular adhesions.
10,11 As an in-office procedure, this is a low-cost treatment option with good reported outcomes (ranges from 50 to 100% success) and favorable side effect profile (7% risk of retinal breaks,
detachment, and progressive VMT).
10Ocriplasmin to treat VMT
A past option was pharmacologic vitreolysis with Ocriplasmin (Jetrea, ThromboGenics). Ocriplasmin was an intravitreal injection that led to enzymatic cleavage of the posterior vitreous cortex from the ILM that could provide pharmacologic resolution of VMT (26% resolution of VMT with ocriplasmin versus 10% resolution with placebo).12
However, there remained a concern around serious but rare complications of
retinal detachment, retinal tears, severe transient vision loss, and severe long-standing vision loss with photoreceptor atrophy. This, along with the cost ($3,950) and the possible need for vitrectomy despite treatment with Ocriplasmin, limited its use among providers.
11 In late May 2020, the manufacturing and distribution of Jetrea were discontinued by ThromboGenics for business reasons in the United States.
Full-thickness macular hole (FTMH)
FTMH, per the IVTS, are anatomic openings in the fovea that involve the entire neurosensory retina. Patients with a
full-thickness macular hole often present symptomatically with reduced visual acuity, metamorphopsias, and scotomas. Less commonly, they may present asymptomatically. FTMH clinically appears as circular red regions in the fovea, but small FTMH may be difficult to visualize clinically.
FTMH would represent Gass stages 2 to 4, depending on the size and presence/absence of the Weiss ring. The IVTS grading system uses OCT-classified FTMH based on their size.
Table 3 compares Gass and IVTS staging for classifying full-thickness macular holes.
| Gass Staging | IVTS Staging | Comments |
|---|
| Stage 0 | VMA | Normal consequence of aging. |
| Stage 1A and 1B | VMT | Vitreous traction with no FTMH. |
| Stage 2 | FTMH (small or medium) without Weiss ring | Stage 2 holes per Gass classification were those <400μm without the presence of Weiss ring. This encompasses both small (≤250μm) and medium (>250μm and ≤400μm) sized holes per the IVTS as long as they do not have the presence of Weiss ring. |
| Stage 3 | FTMH (large) without Weiss ring | Stage 3 holes per Gass were classified as those ≥400μm without Weiss ring. This would encompass large-sized FTMH without Weiss ring on IVTS. |
| Stage 4 | FTMH (small, medium, or large) with Weiss ring | The Gass classification for stage 4 hole did not require a size, but only the presence of Weiss ring. Any sized IVTS hole with a Weiss ring would meet this category. |
Per the IVTS, holes should be measured at their narrowest opening in the mid-retina with a line drawn parallel to the RPE. You should not include the operculum in the measurement of the hole. When measuring macular holes, you must ensure that you are using the
OCT cross-section that best represents the size of the hole at its largest diameter.
The reason for staging FTMH in this manner is that the hole size has implications on management recommendations and outcomes. Smaller-sized macular holes and those with less chronicity (generally cited as less than 6 months) have a higher chance of successful surgical closure and better visual acuity outcomes.1
Break down of the spectrum of FTMH size and treatment recommendations:
- Small FTMHs are less than 250μm in diameter.1 There is a low chance of spontaneous closure of small FTMH, particularly those with residual vitreous traction or with the recent release of vitreous traction; however, most progress to larger-sized holes.11 There is a high chance of successful surgical closure (nearly 100%). They also have the highest success with pharmacologic vitreolysis with ocriplasmin.1
- Medium FTMHs are between 250 and 400μm in diameter. They have a greater than 90% chance of successful surgical closure with or without peeling of the ILM. Success with pharmacologic vitreolysis has been reported but is lower with the medium than small FTMH.1
- Large-sized FTMHs are greater than 400μm in diameter. Successful surgical closure has been reported to be between 90 and 95% with ILM peeling and only 75% without. Successful hole closure has not been reported with pharmacologic vitreolysis.1,4
FTMH should also be classified in regard to the presence or absence of vitreous traction. Only those with residual vitreous traction are candidates for pharmacologic vitreolysis.1
Primary vs. secondary macular holes
Macular holes may also be categorized as primary or secondary. This article focuses on primary macular holes, also described in the literature as idiopathic macular holes. These are macular holes that develop from vitreous traction during the anomalous PVD events described above. Macular holes that occur through other mechanisms, such as blunt force trauma or macular atrophy (as in macular telangiectasia type 2), are called secondary macular holes.1
In Figure 7, a 21-year-old female patient presents with macular hole from head trauma related to a car accident. The patient also has
subretinal hemorrhage and choroidal rupture. This would be considered a secondary macular hole.
Figure 7: Courtesy of Jessica Haynes, OD
Pneumatic vitreolysis is also a treatment option for some FTMH. Success is more likely with smaller-sized holes, as such pneumatic vitreolysis may be considered for small to medium-sized holes. Successful hole closure with small macular holes is reported between 50 to 90%. Additional characteristics of ideal candidates would be presence of focal vitreomacular adhesions and the absence of epiretinal retinal membrane.10,11
Epiretinal membrane (ERM)
As the posterior vitreous cortex separates from the macular surface, microscopic regions of damage can lead to glial cell proliferations and the formation of ERM. These membranes may form after fully separating the vitreous cortex or may be present alongside VMA and VMT. They can also be seen concomitantly with FTMH, LMH, and per the IVTS, are a distinguishing feature of macular pseudoholes.1
“On OCT, ERM present as thin hyper-reflective films on the retinal surface.”
As ERM form, they can contract and lead to foveal thickening, intraretinal or subfoveal cystic spaces, distorted foveal pit, macular schisis, LMH, and macular striae. Patients can present asymptomatically or may have visual symptoms, including reduced visual acuity, decreased contrast sensitivity, and metamorphopsia.
In Figure 8, the top image illustrates ERM creating macular striae but not affecting the foveal contour/structure in an asymptomatic patient. In the bottom image, ERM is creating macular striae, macular thickening, and loss of the foveal pit in a symptomatic patient.
Figure 8: Courtesy of Jessica Haynes, OD
Surgical intervention involves vitrectomy with peeling of the ERM and possibly peeling of the ILM. There is no uniform agreement on when surgery should be considered. Some surgeons require a specific visual acuity reduction, such as 20/40 or worse, while others may prefer to base intervention on patient symptoms rather than visual acuity alone.
It is important to remember that patients can have good visual acuity while exhibiting impactful symptoms such as metamorphopsia and reduced contrast sensitivity. Conversely, patients may have reduced visual acuity and remain entirely asymptomatic.
Lamellar macular hole (LMH)
LMH are partial thickness foveal defects. They do not affect the full foveal thickness like FTMH. A lamellar macular hole is often difficult to detect on the clinical examination, but careful examination may reveal distortion or absence of the foveal light reflex, oval-shaped
reddish lesion in the fovea, or cystic appearance of the fovea.
Patients may be asymptomatic or present with symptoms similar to those with VMT. Visual acuity often remains good, especially if there is a lack of photoreceptor involvement, and LMH is typically slowly progressive or non-progressive.1
On OCT, LMH can exhibit any of the following signs per the IVTS:
- An irregular foveal contour
- A defect in the inner fovea (may not have actual loss of tissue)
- Intraretinal splitting (schisis), typically between the outer plexiform and outer nuclear layers (seen in Figure 8)
Figure 9 represents a variety of LMH presentations. The top left is distorted/altered foveal contour, and the top right is TLH with macular schisis and subtle ERM (red arrow). The bottom left image features DLH with LHEP (blue arrows), and the bottom right is LMH with ERM, residual VMT, and concurrent AMD.
Figure 9: Courtesy of Jessica Haynes, OD
Developments in the definition of LMH
Traditionally LMH were thought to be formed by either the anterior-posterior forces of previous or concomitant VMT or from centripetal forces of epiretinal membranes that split apart the retinal layers mechanically.1
More recently, discussion has shifted to consider that the term LMH may encompass two separate pathological processes. It has been proposed that the formation of LMH can result from a tractional component, tractional lamellar hole (TLH), as described above, or from a degenerative process, degenerative lamellar hole (DLH), where the foveal alterations and cavitations are not created by sheer tractional force but by a degenerative process.13,14
A closer look at the degenerative lamellar hole
The exact pathogenesis of DLH is unclear, but it is thought that foveal disruption from prior forces such as VMT creates tractional disruption and damage to Müller cells, which results in tissue atrophy causing intraretinal cavitations, photoreceptor atrophy, and formation of unique epiretinal membranes called lamellar hole-associated epiretinal proliferation (LHEP).13,14
Unlike the thin hyper-reflective bands on the surface of the retina that are seen on
optical coherence tomography with ERM, LHEP presents as thicker bands with medium reflectivity. DLH can form following foveal disruption from VMT, CME, ERM, and even after successful FTMH closure. DLH is often stable or slowly progressive but can progress to FTMH. The tractional forces that ultimately lead to DLH can precede the formation of DLH by months to years.
13,14There are no standard recommendations for surgical intervention for LMH, with reports of visual improvement ranging from only 25% with surgery to 75%.1 Some of the visual improvement may be related to the removal of concomitant ERM. Visual prognosis for those with DLH may be worse due to foveal atrophy, which can include photoreceptor atrophy.13-15 Patients should be carefully selected and counseled due to these visual limitations.
Macular pseudohole
Unlike the previous terminology, the term macular pseudohole is a non-OCT term based on the clinical slit lamp examination. Essentially, this word is used to describe a
fundoscopic appearance that looks like an FTMH but is not: hence
pseudohole. As there is no FTMH, visual acuity is often good. OCT will confirm the absence of an FTMH.
The IVTS describes that a macular pseudohole on OCT has all of the following characteristics:
- “Invaginated or heaped foveal edges
- Concomitant ERM with central opening
- Steep macular contour to the central fovea with near-normal central foveal thickness
- No loss of retinal tissue”
Per their definition, the term macular pseudohole should be used in the presence of an accompanying ERM. Traction from the ERM creates the heaped and distorted foveal edges, giving the pseudohole appearance. Since visual acuity is often good, pseudoholes can frequently be monitored. If there is visual decline from the associated ERM, vitrectomy with membrane peeling is often successful.1
Figure 10 presents imaging from a patient with fine glistening ERM on the macular surface and round, reddish distortion at the fovea on clinical exam mimicking at FTMH. OCT confirms that this is a pseudohole with ERM creating heaped foveal margins and no FTMH.
Figure 10: Courtesy of Jessica Haynes, OD
Imaging recommendations for VMI disease
The clinical examination,
patient history, including visual symptoms, and ancillary testing, such as the Amsler grid or evaluation of the Watzke-Allen sign, remain important in diagnosing patients with VMI disease. However, OCT is the most critical component of identifying and staging macular holes, and OCT has become the standard of care when managing these conditions.
When evaluating OCT imaging in a patient with VMI disease, it is important to look through all of the OCT cross-section scans. Pathological changes can exist on just one OCT cross-section—changing a diagnosis or management plan.
When considering what type of OCT image to obtain, prioritize high-resolution scans over low-resolution scans and consider scanning in a radial pattern centered on the fovea to obtain more cuts through the fovea. If radial scanning patterns are not available, consider denser cube scans that provide higher sampling of the fovea.
Figure 11 is a 24-line radial OCT obtained from a 74-year-old female with FTMH. Each slice of the OCT shows differing anatomy. The size of the macular hole varies based on the scan that is viewed. In addition, only a few scans show the presence of residual vitreous traction (arrow), which could be important in treatment recommendations. All scans must be viewed to ensure a proper diagnosis.
Figure 11: Courtesy of Jessica Haynes, OD
When interpreting the OCT image, consider the following:
- Evaluate the status of the posterior vitreous cortex. Is there lifting of the posterior vitreous cortex from the macular surface or complete separation of the posterior vitreous cortex from the macular surface?
- If there is lifting of the posterior vitreous cortex from the macular surface, is it altering the macular anatomy? If it is not, then this would represent VMA. If it is, the patient may have VMT or FTMH with residual vitreous attachment.
- To distinguish VMT from FTMH, consider if there is a full-thickness break in all layers of the neurosensory retina. If there is a full-thickness break in all layers of the neurosensory retina in any OCT cross-section scan, then the patient has an FTMH.
- If there is full thickness macular hole, measure the narrowest opening in the mid-retina (excluding the operculum) to determine the size of the hole (small ≤250μm, medium >250μm and ≤400μm, or large >400μm).
- Be sure to look through all OCT images to ensure you are measuring the narrowest opening in the center of the hole (e.g., the middle of the retina at its narrowest opening using a scan that cuts through the center of the hole along the widest diameter). Staging the hole based on size can help to determine the best treatment options and predict patient outcomes.
- If there is not an FTMH, but there is vitreous traction altering the macular anatomy, this is a VMT. An LMH may also be present concomitantly with VMT, as shown in previous images.
- Consider on the scan if the VMT is focal or broad and how the VMT is altering the macula. While VMT can spontaneously resolve, focal and broad VMT tend to lead to different outcomes. Keep in mind that a VMT can be progressive and evolve into an FTMH.
- Consider if there is the presence of an ERM on the OCT image. This can alter the best treatment recommendations.
- When considering the diagnosis of FTMH, consider if the patient may have an LMH or macular pseudohole based on the OCT imaging. While patients with FTMH generally progress to larger hole formation and have worse visual outcomes with delayed intervention, patients with LMH and macular pseudohole may retain good visual acuity and not require treatment.
Conclusion
Consistent use of standard terminology and staging of VMI disease will improve
inter-provider communications and enhance patient outcomes. Accurately staging VMI disease will help providers understand treatment options and better educate patients regarding potential visual outcomes.